Abstract
OBJECTIVE: Although temporal lobe epilepsy (TLE) is recognized as a system-level disorder, little work has investigated pathoconnectomics from a dynamic perspective. By leveraging computational simulations that quantify patterns of information flow across the connectome, we tested the hypothesis that network communication is abnormal in this condition, studied the interplay between hippocampal- and network-level disease effects, and assessed associations with cognition.
METHODS: We simulated signal spreading via a linear threshold model that temporally evolves on a structural graph derived from diffusion-weighted magnetic resonance imaging (MRI), comparing a homogeneous group of 31 patients with histologically proven hippocampal sclerosis to 31 age- and sex-matched healthy controls. We evaluated the modulatory effects of structural alterations of the neocortex and hippocampus on network dynamics. Furthermore, multivariate statistics addressed the relationship with cognitive parameters.
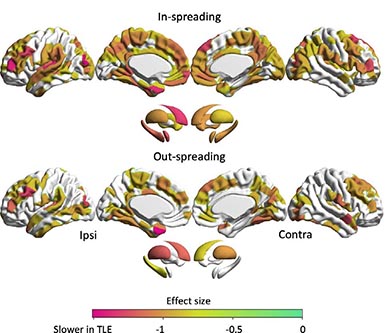
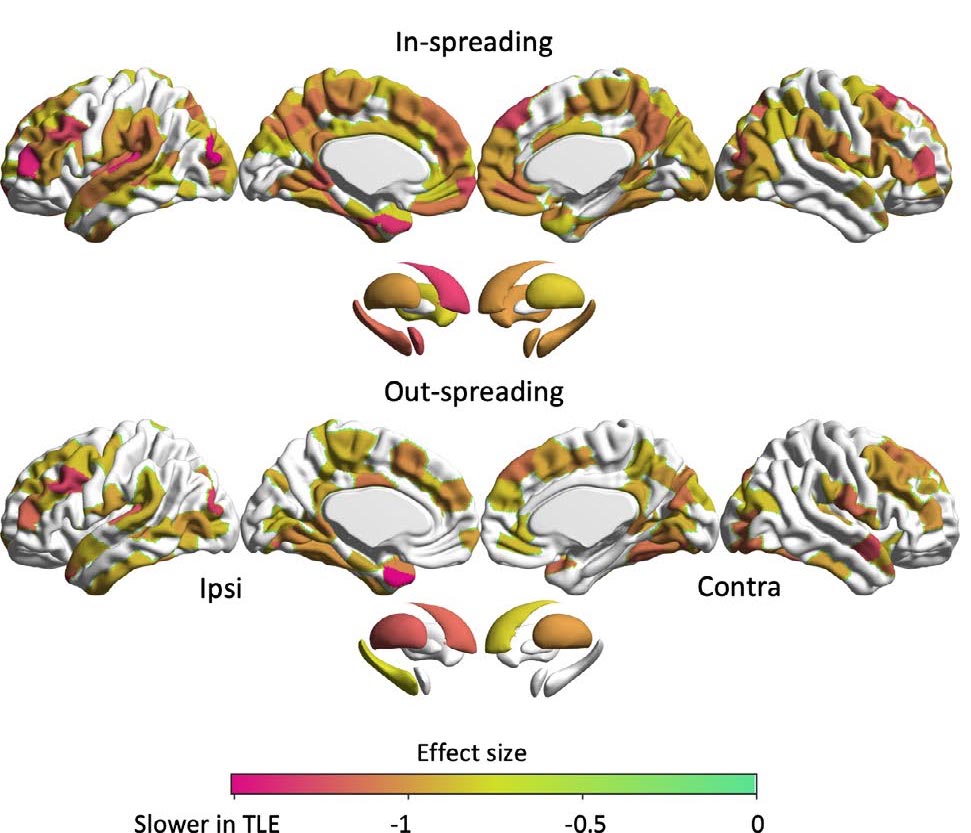
RESULTS: We observed a slowing of in- and out-spreading times across multiple areas bilaterally, indexing delayed information flow, with the strongest effects in ipsilateral frontotemporal regions, thalamus, and hippocampus. Effects were markedly reduced when controlling for hippocampal volume but not cortical thickness, underscoring the central role of the hippocampus in whole-brain disease expression. Multivariate analysis associated slower spreading time in frontoparietal, limbic, default mode, and subcortical networks with impairment across tasks tapping into sensorimotor, executive, memory, and verbal abilities.
SIGNIFICANCE: Moving beyond descriptions of static topology toward the formulation of brain dynamics, our work provides novel insight into structurally mediated network dysfunction and demonstrates that altered whole-brain communication dynamics contribute to common cognitive difficulties in TLE.